

A short list of some of the combinations of gases and their colours is below, but there are many more to choose from.Ĭoating and baking into the tubes of fluorescent powders can also help to create different shades of these colours too, and it is also possible to use coloured tubes for this purpose.ĭue to the increasing interest in neon, it is not only now used as advertising, but also as a form of artwork. The colour we see on signs everywhere today, is created via the different gases combined with the neon in the tubes. When these atoms are restored to their usual energy level, they release a ‘photon’ (particle of light) and emit visible light as a consequence. When these atoms bounce around the tube they collide with each other, raising the energy levels contained within the neon molecules. These ions and electrons then spread themselves throughout the entire tube effectively ensuring that the gas fills the tube in its entirety. However, by passing an electric current through the tube with kinetic energy, the atoms in the molecules of the neon become excited and release electrons into the gas. Because of this, neon can be placed into a sealed tube, where it will start to drift around without causing any pressure build-up.

In 1923, Packard motors immediately commandeered two of the inventor’s early neon signs and began to use them for advertising purposes. Thus, the idea of neon signage was born.Īs neon is an inert (or noble) gas, meaning that it is a naturally unreactive element within the Earth’s atmosphere and can be easily stored. However, Georges Claude furthered this by discovering that neon gases (when combined with other elements and fed with electrical charges) could display a variety of bright, colourful lights when an electrical discharge was applied to a sealed tube of neon gas.Ĭlaude displayed the first neon lamp that he created to the general public an expo in Paris in 1910, before patenting the neon lighting tube in 1915 for commercialisation. The first tube to contain neon was made for a scientific study into applying electrical discharges to different gases and various other substances. They discovered microscopic quantities of the gas occur naturally in our atmosphere. Who invented neon signs?Ī French inventor, Georges Claude, invented neon signs in 1910 but neon itself was actually discovered in 1898 in gas form by William Ramsay and Morris Travers. With that in mind, we’ve compiled some interesting facts about neon, and how it’s use for signage has developed over the years. There’s something about neon that really attracts the attention of passers-by, young and old and says “Hey, come in, see what we’re doing!” However, not everyone knows what causes the neon to glow so brightly, and how it can emit different colour light. Show Electronic configuration of Neon.Neon signs are cool, there’s no doubt about it. no electron is available for bondingĢ) It is mainly use to make switching gears and diving equipmentģ) Neon is important component in cryogenic refrigeratorĥ) It is use in lung diffusion test as a tracker gas
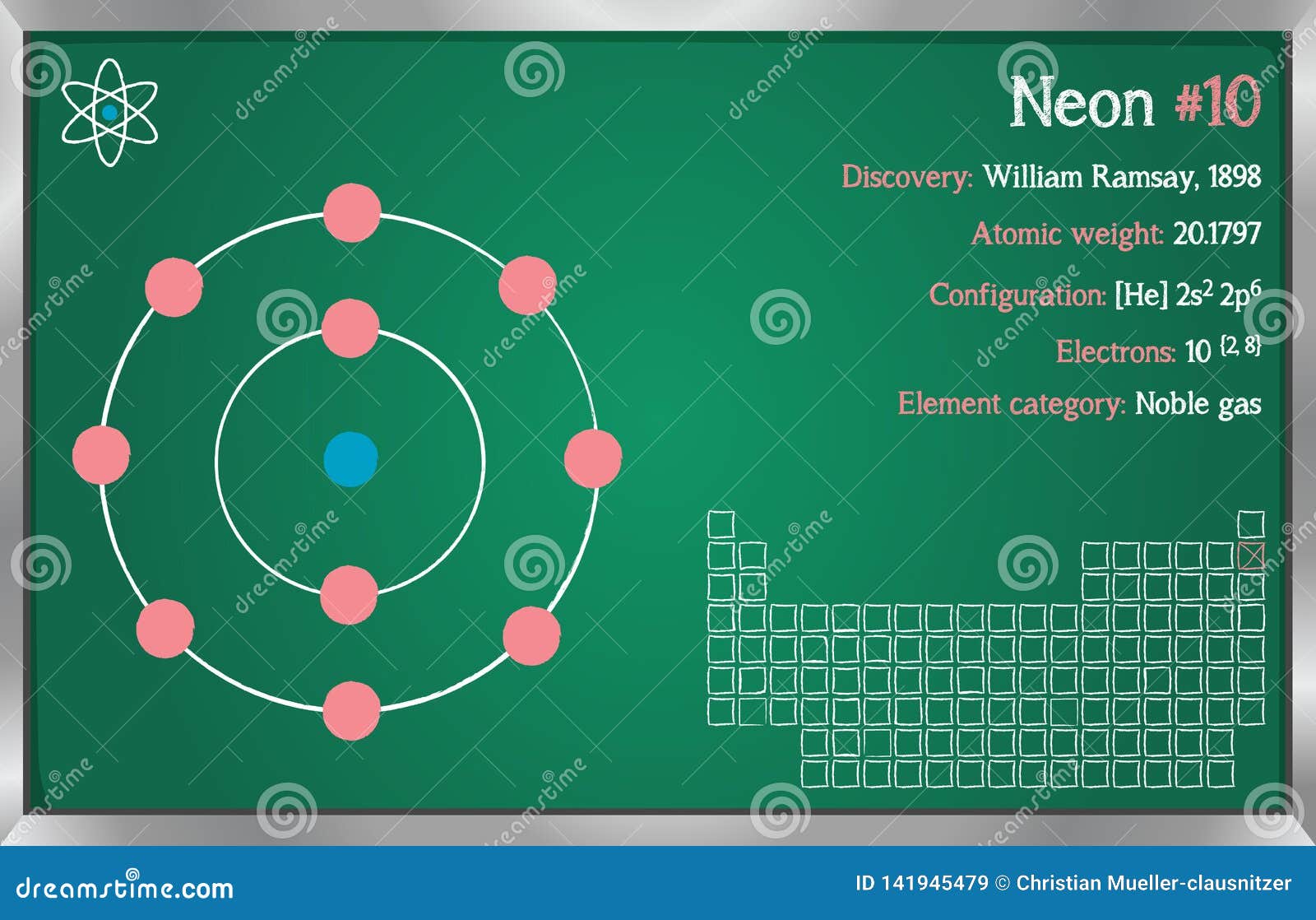
Neon show 0 valency Because it’s octate is completeĤ) Atomic radius : Atomic radius of neon is 38 pmĥ) Reactivity : It is non reactive element because it’s outer most shell is full. Atomic mass is 21Įlectronic configuration of neon is 1S 2 2S 2 2P 6Įlectronic configuration in concert of shell is 2,8Ģ electron present in K shell and 8 electron present in M shell. Atomic mass is 19Ģ1Ne – it is present 0.27%. Origin of name : The name is derived from Greek word’ neos’ Meaning of neos is newġ) Atomic symbol : Atomic symbol of neon is NeĢ) Atomic number Atomic number of neon is 10ģ) Atomic weight : Atomic weight of neon is 20.17ġ0 proton and 10 neutros present in the neon atomĤ) Position : It is placed in 2 row ( period ) and 18 column( group) It is known as Nobel gas.ģ stable isotopes and remaining are radioactive isotope 20Ne – it is present 90%. neon and halogen is separatedĭiscovery : It is discovered in 1898 by William Ramsay and Morris Travers. It is derived from using fractional distillation. It is 5 th mostly found element in earth surface. When it is burn in tube it glow as a red. It is known as zero gas It is non toxic gas. Neon is an inert gas present in monoatomic from. Atomic Mass, Number, Physical, Chemical properties, Electronic configuration, Valency, Chemical reaction, Uses Neon – Learn all details regarding Neon in Periodic Table i.e.


 0 kommentar(er)
0 kommentar(er)
